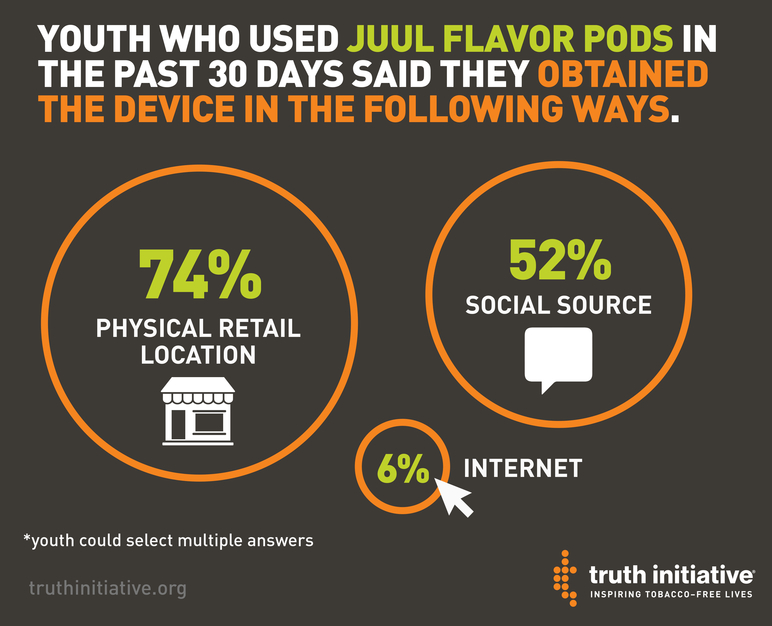
Youth who had used JUUL in the past 30 days reported that they got the product in one or more of these ways:
- Physical retail locations: The most common way youth got JUUL is through physical retail locations. Nearly three quarters — 74 percent — of youth said that they obtained JUUL at a store or retail outlet. In recent weeks, the FDA issued warning letters to retailers, including gas stations, convenience stores and vape shops, for selling the product to minors. The letters warned establishments that an FDA inspector observed a clerk selling the device to a minor, and that “failure to correct these violations may lead to federal enforcement actions, including monetary penalties.”
- Social sources: Just over half — 52 percent — reported that they received JUUL from a social source, such as a friend or family member.
- Online: Although the internet was not the most common way youth obtained JUUL — only 6 percent reported that they received the product through an online transaction — nearly all youth who tried to buy the product online were successful. Among those who attempted an online transaction, 89 percent succeeded. When the FDA issued its first warning letters about JUUL to retailers, it also announced that it contacted eBay to express concerns about JUUL product listings. “We’re thankful for eBay’s swift action to remove the listings and voluntarily implement new measures to prevent new listings from being posted to the web retailer’s site,” FDA Commissioner Scott Gottlieb said in a statement. However, some JUUL products are still available on eBay.
The FDA crackdown on JUUL also includes asking the maker, JUUL Labs, to turn over documents related to marketing, health effects and use among youth. While recent FDA action is encouraging, problems with tobacco products like JUUL won’t be addressed until the FDA fully regulates e-cigarettes and establishes a strong pre-market review process to prevent these kinds of products from being sold in the first place.
Truth Initiative and five other public health and medical groups called on the FDA to take action on JUUL in April. The groups specified five actions, including removing certain JUUL flavors, suspending internet sales and prohibiting branded merchandise.
A Truth Initiative study published in Tobacco Control underscores the problem with youth use of JUUL. It found that 63 percent of young JUUL users did not know that the product always contains the addictive chemical nicotine, and that many reported that use of this product is called "JUULing," indicating that this product is so distinctive, it is perceived as its own category.